INTRODUCTION-PARTITION CHROMATOGRAPHY Partition chromatography can be subdivided into i l iquid-liquid chromatography and i i bonde d-phase chromatography. Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. Analytical method development and validation book.
Analytical Method Development And Validation Book, Analytical Method Development and Validation Paperback 16 May 1997 by Michael E. Books on Google Play Analytical Method Development and Validation Michael E. 38 Handbook of Analytical Validation properties of the analytes for example solubility pKaor pKb spectral properties molecular weight and polarity are used to choose rational starting mobile phase and column conditions from which additional ne-tuning or optimization experiments are carried out. Development and Validation of Plant-derived Medicines for Human.
 Steps For Analytical Method Development Pharmaceutical Guidelines From pharmaguideline.com
Steps For Analytical Method Development Pharmaceutical Guidelines From pharmaguideline.com
Books on Google Play Analytical Method Development and Validation Michael E. Analytical Method Development and Validation Michael E. This book could only be the first step in undestanding of the general requirements for the method validation process. This book is intended to serve as.
Food and Drug Administration.
Read another article:
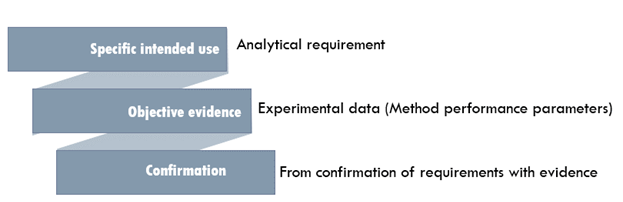
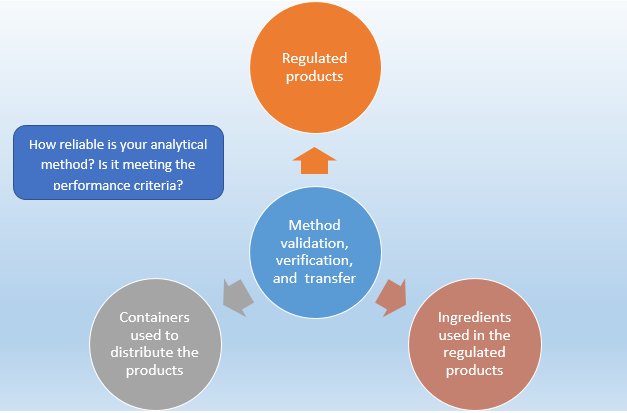
Method validation is defined as the process of pro ving that an analytical technique is acceptable for the intended use and this is an important. Analytical Procedures and Methods Validation for Drugs and Biologics Guidance for Industry. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. The need to validate an analytical or bioanalytical method is encountered by analysts in the pharmaceutical industry on an almost daily basis because adequately validated methods are a necessity for approvable regulatory filings. Food and Drug Administration.
 Source: semanticscholar.org
Source: semanticscholar.org
This book could only be the first step in undestanding of the general requirements for the method validation process. Food and Drug Administration. Development and Validation of Plant-derived Medicines for Human. 67-83 Chantal Incledon. Pdf Step By Step Analytical Methods Validation And Protocol In The Quality System Compliance Industry Semantic Scholar.
 Source: researchgate.net
Source: researchgate.net
Develop newer analytical methods for such drug s. Sunday Feb 7 Details Save Extra with 4 offers. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation. Next the process of validation is discussed starting with instrument qualification and concluding with system suitability. Pdf Method Development And Validation Skills And Tricks.
 Source: elsevier.com
Source: elsevier.com
This book is intended to serve as a guide to the analyst in terms of the issues and parameters that must be considered in the development and validation of analytical methods. Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research Dehradun Uttarakhand India 1. What constitutes a validated method however is subject to analyst interpretation because there is no universally accepted industry practice for assay validation. 2009-03-15 Analytical Method Development and Validation by Michael E. Handbook Of Analytical Quality By Design 1st Edition.
 Source: researchgate.net
Source: researchgate.net
Santhosh GNagasowjanya AAjitha YUma Maheswara Rao Department of Pharmaceutical Analysis and Quality Assurance CMR College of Pharmacy Medchal Road Kandlaykoya. Development and Validation of Plant-derived Medicines for Human. Shedding light on method validation from a practical standpoint the handbook. Books on Google Play Analytical Method Development and Validation Michael E. Pdf Development And Validation Of Rp Hplc Method For The Estimation Of Lisinopril In Tablet Dosage Form.
 Source: semanticscholar.org
Source: semanticscholar.org
Ad Über 7 Millionen englische Bücher. Written for practitioners in both the drug and biotechnology industries the Handbook of Analytical Validation carefully compiles current regulatory requirements on the validation of new or modified analytical methods. Swartz Editor Ira S. This book could only be the first step in undestanding of the general requirements for the method validation process. Pdf Step By Step Analytical Methods Validation And Protocol In The Quality System Compliance Industry Semantic Scholar.
 Source: complianceonline.com
Source: complianceonline.com
Method Development and Validation of Analytical Procedures Kapil Kalra Dev Bhoomi Institute of Pharmacy an d Research Dehradun Uttarakhand India 1. The need to validate an analytical or bioanalytical method is encountered by analysts in the pharmaceutical industry on an almost daily basis because adequately validated methods are a necessity for approvable regulatory filings. In addition to the critical issues surrounding method validation this book also deals with other related factors such as method development data acquisition automation cleaning validation and regulatory considerations. In Analytical Method Development and Validation the subject of developing and optimizing an HPLC method is presented culminating in a step-by-step guideline. Analytical Method Validation Questions And Answers.
 Source: pinterest.com
Source: pinterest.com
Sunday Feb 7 Details Save Extra with 4 offers. Vol 4 Issue 4 2014 274-280. Department of Health and Human Services. Method validation is defined as the process of pro ving that an analytical technique is acceptable for the intended use and this is an important. Pin On Best Sample Template.
 Source: complianceonline.com
Source: complianceonline.com
Analytical Method Development and Validation Paperback 16 May 1997 by Michael E. Ad Über 7 Millionen englische Bücher. Development and Validation of Automated Methods Pages. The need to validate an analytical or bioanalytical method is encountered by analysts in the pharmaceutical industry on an almost daily basis because adequately validated methods are a necessity for approvable regulatory filings. Analytical Method Validation Verification And Transfer Right.
 Source: americanpharmaceuticalreview.com
Source: americanpharmaceuticalreview.com
This book could only be the first step in undestanding of the general requirements for the method validation process. Requirements to the analyst so as to enab le him to. Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. In Analytical Method Development and Validation the subject of developing and optimizing an HPLC method is presented culminating in a step-by-step guideline. Risk Based Test Method Development Validation And Life Cycle American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology.
 Source: pharmabeginers.com
Source: pharmabeginers.com
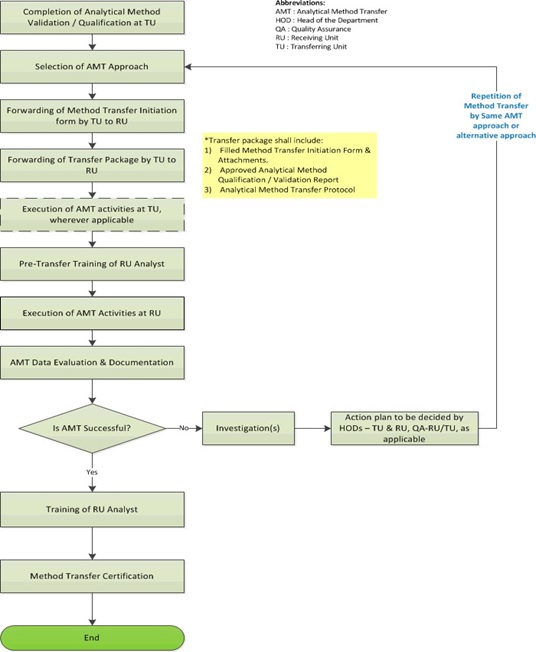
Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Develop newer analytical methods for such drug s. Krull CRC Press May 16 1997 - Science - 96 pages 4 Reviews Describes analytical methods development optimization and validation and provides examples of successful methods development and validation in high-performance liquid chromatography HPLC areas. Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. Analytical Method Transfer Usp 1224 Guideline Pharma Beginners.
 Source: chromatographyonline.com
Source: chromatographyonline.com
Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Method validation is defined as the process of pro ving that an analytical technique is acceptable for the intended use and this is an important. Development and Validation of Plant-derived Medicines for Human. Hplc Methods For Pharmaceuticals.
 Source: sciencedirect.com
Source: sciencedirect.com
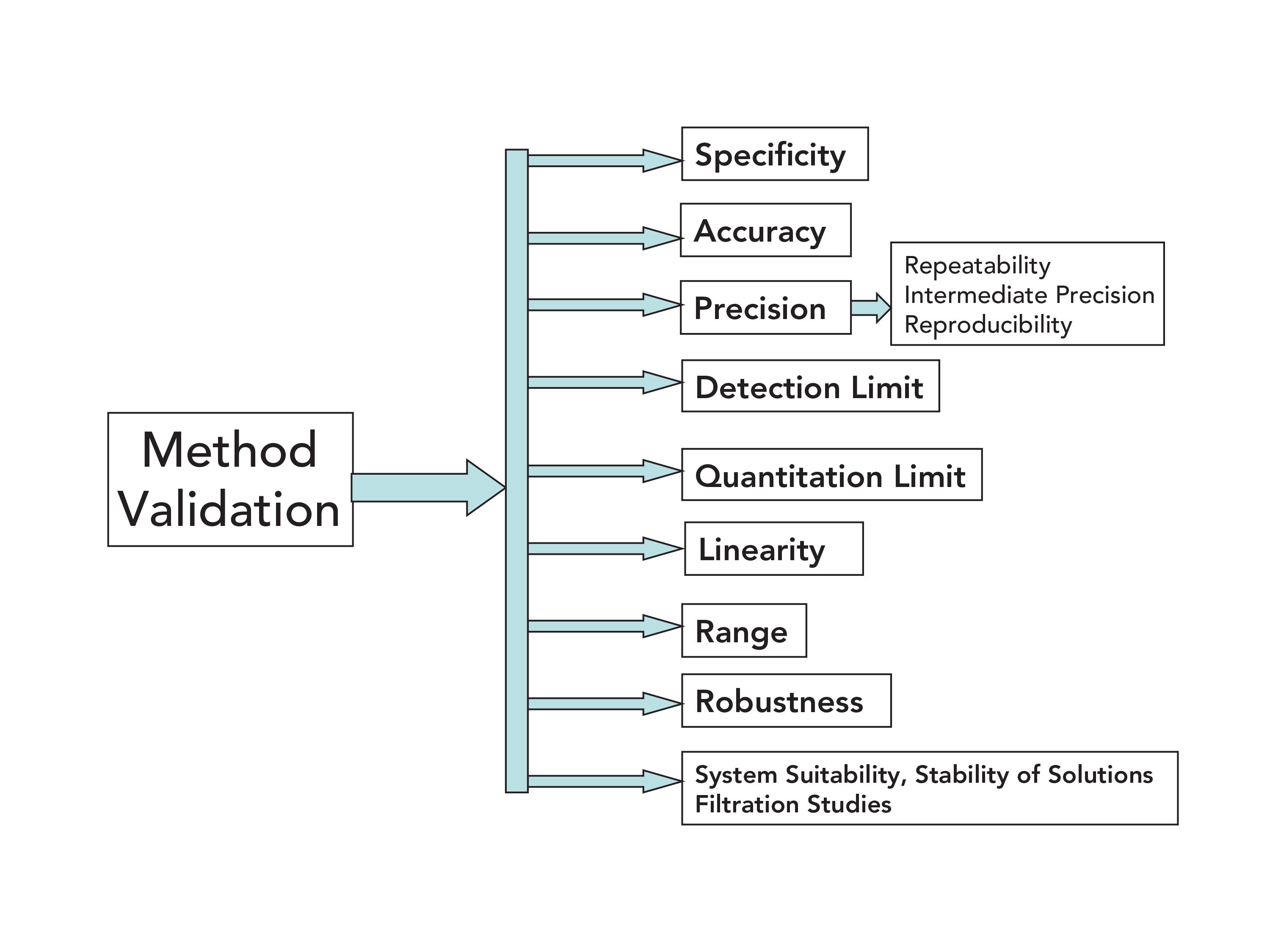
Development and Validation of Automated Methods Pages. Vol 4 Issue 4 2014 274-280. Development there is a need of method validation. Book Description Describes analytical methods development optimization and validation and provides examples of successful methods development and validation in high-performance liquid chromatography HPLC areas. Performance Parameters For Analytical Method Validation Controversies And Discrepancies Among Numerous Guidelines Sciencedirect.
 Source: pinterest.com
Source: pinterest.com
Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Development and Validation of Plant-derived Medicines for Human. Food and Drug Administration. Introduction Method validation is the process used to conf irm that the analytical procedure employed for a specific test is suitable for its intended use. Development And Validation Of Liquid Chromatography Rp Hplc Methodology For Estimation Of Efonidipine Hcl Chemistry Help Teaching Chemistry Science Chemistry.
 Source: pharmaguideline.com
Source: pharmaguideline.com
Krull CRC Press Oct 3 2018 - Science - 96 pages 0 Reviews Describes analytical methods development optimization and. Food and Drug Administration. Krull CRC Press May 16 1997 - Science - 96 pages 4 Reviews Describes analytical methods development optimization and validation and provides examples of successful methods development and validation in high-performance liquid chromatography HPLC areas. Sunday Feb 7 Details Save Extra with 4 offers. Analytical Method Validation Pharmaceutical Guidelines.
 Source: pinterest.com
Source: pinterest.com
To deepen ones knowledge the reader should choose the books eg. Development and Validation of Analytical Methods by Christopher Riley which are focused on explaining the concept from the scientific prospective. Analytical Method Validation and Instrument Performance Verification. Swartz Repost - Removed. Development And Validation Of An Rp Hplc Method For Simultaneous Estimation Of Amlodipine Besy Fundamentals Of Nursing Nursing School Essential Funny Questions.







